What are the atomic masses of the first 30 elements and in the modern periodic table? Hydrogen -1 Helium-4 Lithium = 7 Beryllium = 9 Boron = 11 Carbon = 12 Nitrogen = 14 Oxygen = 16 Fluorine = 19 Neon = 20 Sodium = 23 Magnesium = 24 Aluminum = 27 Silicon = 28 Phosphorus =31 Sulfur = 32 Chlorine =35 Argon = 40 Potassium = 39 Calcium = 40. Mass numbers refer to the numbers of protons and neutrons in the nuclei of specific isotopes. It would be easier to ask for the atomic weights of the first 20 elements on the periodic table. Study Flashcards On Periodic Table Elements by atomic number (1-20) at Cram.com. Quickly memorize the terms, phrases and much more. Cram.com makes it easy to get the grade you want! Hydrogen -1 Helium-4 Lithium = 7 Beryllium = 9 Boron = 11 Carbon = 12 Nitrogen = 14 Oxygen = 16 Fluorine = 19 Neon = 20 Sodium = 23 Magnesium = 24 Aluminum = 27 Silicon = 28 Phosphorus =31 Sulfur = 32 Chlorine =35 Argon = 40 Potassium = 39 Calcium.
Create a chart detailing key information about the first 20 elements. Click 'Start Assignment'. First find the symbol and names on the first 20 elements and use Textables to write them in each cell in the correct order. Remember if an element has two letters for its symbol, the first letter will be a capital and the second will be lowercase.

List of first 50 elements of the periodic table by atomic number including the chemical symbol and the atomic weight. You can print the list of elements by hitting the print button below.
Atomic And Mass Number Of First 20 Elements
| Atomic Number | Chemical Symbol | Element Name | Atomic Weight (u) |
|---|---|---|---|
| 1 | H | Hydrogen | 1.008 |
| 2 | He | Helium | 4.002602 |
| 3 | Li | Lithium | 6.94 |
| 4 | Be | Beryllium | 9.0121831 |
| 5 | B | Boron | 10.81 |
| 6 | C | Carbon | 12.011 |
| 7 | N | Nitrogen | 14.007 |
| 8 | O | Oxygen | 15.999 |
| 9 | F | Fluorine | 18.99840316 |
| 10 | Ne | Neon | 20.1797 |
| 11 | Na | Sodium | 22.98976928 |
| 12 | Mg | Magnesium | 24.305 |
| 13 | Al | Aluminium | 26.9815385 |
| 14 | Si | Silicon | 28.085 |
| 15 | P | Phosphorus | 30.973762 |
| 16 | S | Sulfur | 32.06 |
| 17 | Cl | Chlorine | 35.45 |
| 18 | Ar | Argon | 39.948 |
| 19 | K | Potassium | 39.0983 |
| 20 | Ca | Calcium | 40.078 |
| 21 | Sc | Scandium | 44.955908 |
| 22 | Ti | Titanium | 47.867 |
| 23 | V | Vanadium | 50.9415 |
| 24 | Cr | Chromium | 51.9961 |
| 25 | Mn | Manganese | 54.938044 |
| 26 | Fe | Iron | 55.845 |
| 27 | Co | Cobalt | 58.933194 |
| 28 | Ni | Nickel | 58.6934 |
| 29 | Cu | Copper | 63.546 |
| 30 | Zn | Zinc | 65.38 |
| 31 | Ga | Gallium | 69.723 |
| 32 | Ge | Germanium | 72.63 |
| 33 | As | Arsenic | 74.921595 |
| 34 | Se | Selenium | 78.971 |
| 35 | Br | Bromine | 79.904 |
| 36 | Kr | Krypton | 83.798 |
| 37 | Rb | Rubidium | 85.4678 |
| 38 | Sr | Strontium | 87.62 |
| 39 | Y | Yttrium | 88.90584 |
| 40 | Zr | Zirconium | 91.224 |
| 41 | Nb | Niobium | 92.90637 |
| 42 | Mo | Molybdenum | 95.95 |
| 43 | Tc | Technetium | 98 |
| 44 | Ru | Ruthenium | 101.07 |
| 45 | Rh | Rhodium | 102.9055 |
| 46 | Pd | Palladium | 106.42 |
| 47 | Ag | Silver | 107.8682 |
| 48 | Cd | Cadmium | 112.414 |
| 49 | In | Indium | 114.818 |
| 50 | Sn | Tin | 118.71 |
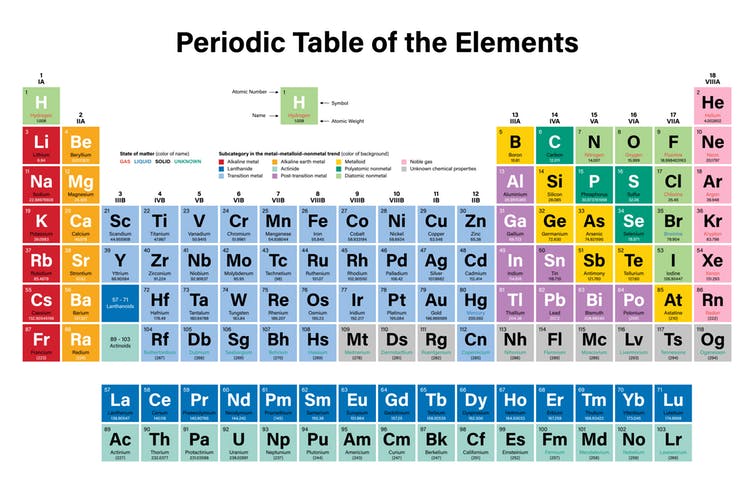


In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
Atomic Number And Mass Number Of First 20 Elements Pdf
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.
