June 2018 Chemistry Regents #1-5
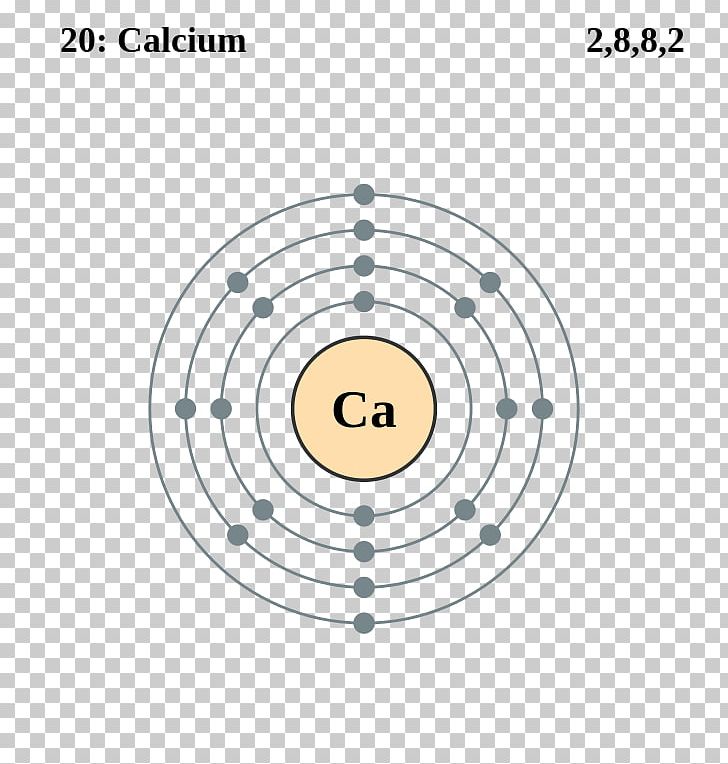
Electrons In Calcium 43
Highlight to reveal answers and explanations
This can be memorized, or found on the periodic table. Calcium is the 20th element, with 20 protons (since the number of protons directly changes the element itself). Since a stable atom has a net charge of 0, we must have 20 electrons. Name: Calcium Symbol: Ca Atomic Number: 20 Atomic Mass: 40.078 amu Melting Point: 839.0 °C (1112.15 K, 1542.2 °F) Boiling Point: 1484.0 °C (1757.15 K, 2703.2 °F) Number of Protons/Electrons: 20 Number of Neutrons: 20 Classification: Alkaline Earth Crystal Structure: Cubic Density @ 293 K: 1.55 g/cm 3 Color: Silvery Atomic Structure. Calcium is the first (lightest) element to have six naturally occurring isotopes. Calcium-40 is composed of 20 protons, 20 neutrons, and 20 electrons. By far the most common isotope of calcium in nature is 40Ca, which makes up 96.941% of all natural calcium.
Questions | Answer | Links | Explanations |
| 1 Which statement describes the charge and location of an electron in an atom? (1) An electron has a positive charge and is located outside the nucleus. (2) An electron has a positive charge and is located in the nucleus. (3) An electron has a negative charge and is located outside the nucleus. (4) An electron has a negative charge and is located in the nucleus. | 3 | electrons..negative..outside nucleus protons..positive..in nucleus | |
| 2 Which statement explains why a xenon atom is electrically neutral? (1) The atom has fewer neutrons than electrons. (2) The atom has more protons than electrons. (3) The atom has the same number of neutrons and electrons. (4) The atom has the same number of protons and electrons. | 4 | neutral..postive equals negative protons equal electrons | |
| 3 If two atoms are isotopes of the same element, the atoms must have (1) the same number of protons and the same number of neutrons (2) the same number of protons and a different number of neutrons (3) a different number of protons and the same number of neutrons (4) a different number of protons and a different number of neutrons | 2 | isotopes..same number protons different number of neutrons | |
| 4 Which electrons in a calcium atom in the ground state have the greatest effect on the chemical properties of calcium? Bria mac download. (1) the two electrons in the first shell (2) the two electrons in the fourth shell (3) the eight electrons in the second shell (4) the eight electrons in the third shell | 2 | valence electrons are the ones that determine chemical properties highest energy level electrons | |
| 5 The weighted average of the atomic masses of the naturally occurring isotopes of an element is the (1) atomic mass of the element (2) atomic number of the element (3) mass number of each isotope (4) formula mass of each isotope | 1 | definition of atomic mass |
June 2018 Chemistry Regents #1-5

Highlight to reveal answers and explanations


Number Of Protons In Calcium
Questions | Answer | Links | Explanations |
| 1 Which statement describes the charge and location of an electron in an atom? (1) An electron has a positive charge and is located outside the nucleus. (2) An electron has a positive charge and is located in the nucleus. (3) An electron has a negative charge and is located outside the nucleus. (4) An electron has a negative charge and is located in the nucleus. | 3 | electrons..negative..outside nucleus protons..positive..in nucleus | |
| 2 Which statement explains why a xenon atom is electrically neutral? (1) The atom has fewer neutrons than electrons. (2) The atom has more protons than electrons. (3) The atom has the same number of neutrons and electrons. (4) The atom has the same number of protons and electrons. | 4 | neutral..postive equals negative protons equal electrons | |
| 3 If two atoms are isotopes of the same element, the atoms must have (1) the same number of protons and the same number of neutrons (2) the same number of protons and a different number of neutrons (3) a different number of protons and the same number of neutrons (4) a different number of protons and a different number of neutrons | 2 | isotopes..same number protons different number of neutrons | |
| 4 Which electrons in a calcium atom in the ground state have the greatest effect on the chemical properties of calcium? (1) the two electrons in the first shell (2) the two electrons in the fourth shell (3) the eight electrons in the second shell (4) the eight electrons in the third shell | 2 | valence electrons are the ones that determine chemical properties highest energy level electrons | |
| 5 The weighted average of the atomic masses of the naturally occurring isotopes of an element is the (1) atomic mass of the element (2) atomic number of the element (3) mass number of each isotope (4) formula mass of each isotope | 1 | definition of atomic mass |
