Miller Electric Gas Regulators are designed to provide control of gases such as acetylene, oxygen, liquefied propane, argon, carbon dioxide, helium, and more. Helium is an inert gas that among other things is used medically to alleviate the symptoms of airway obstruction, as part of a diving mix in deep-sea diving or as balloon gas.

Helium, along with neon, argon, krypton, xenon, and radon, is an inert gas (also known as a noble gas) – that is, it does not normally react with other elements to form chemical compounds. Unlike most of these, however, helium is, at least for us, a very common gas. Despite lacking the usual property of such gases – eight electrons in its outer atomic shell – helium is still classified as an inert gas because its outer shell, which holds just two electrons, is still full.
THE INERT/NOBLE GASES

Inert Helium Core
The discovery of helium during the 1800s paralleled the discovery of the inert gases more generally. Beginning with Henry Cavendish in the late 1700s, early chemists realized that air contained small amounts of a special gas which did not participate in any chemical reactions. In the late 1800s, further research by John Strutt Lord Rayleigh confirmed the existence of this gas, and called it argon, borrowing as inspiration a Greek term for “inactive.” Shortly afterward, William Crookes identified another non-reacting gas given off by uranium samples, and called it helium.
Why Is Helium Inert
In chemical terms, we now understand why these mysterious gases did not react as expected. The “inert gases” – since renamed noble gases, because under extremely specific conditions they can actually be made to react actively with other chemicals – are six gases with no colour and no smell: helium, neon, argon, krypton, xenon, and radon. To understand why they behave this way, it is necessary to delve very deeply into atomic theory.
Atoms contain three basic building blocks: protons and neutrons which are packed very closely together in a dense nucleus, and electrons which zip around the nucleus at high speed. Although the paths these electrons travel are effectively random, rather than the coherent orbits described in the early twentieth century by Ernest Rutherford (which still form the basis for much high school-level chemistry), it is still useful to think of these electrons as sticking within distinct orbits or “shells,” of varying distance from the nucleus of their atom. Usually the outermost shell can safely hold up to eight electrons, although with hydrogen and helium (because of their extremely small nuclei), the outer shell holds only two electrons.
Ionic bonds form between elements, creating chemical compounds, when atoms with partially empty outer shells come together. Some elements (those on the left of the periodic table) tend to give off free electrons, reducing their number in the outermost shell until it is entirely empty; those on the right of the periodic table, except for the noble gases themselves, tend to take electrons from other atoms, filling up their outer shell entirely. This leads to the formation of such commonly known compounds as table salt (sodium chloride), in which sodium gives up one electron and chlorine gains one electron.
Inert gases, however, already have full outer shells of electrons. Consequently, they tend neither to give away free electrons, nor to take electrons from other atoms. For quite some time, it was believed that it would be entirely impossible to create compounds from noble gases. Scientists now realize that it is difficult but not impossible, under carefully controlled conditions. Specifically, a number of compounds have been created using the heavier noble gases. However, no confirmed compounds currently exist containing either helium or neon.
HELIUM AS A NOBLE/INERT GAS
Helium Gas Prices
.png?itok=AxgOWZuE)
All other noble or inert gases operate on an effectively identical premise: their outer shell is capable of holding eight electrons, and it holds electrons – and, therefore, it neither takes nor gives up any electrons when mixing with other chemicals. Helium is slightly different: its outer shell is only capable of holding two electrons. However, it is still filled to capacity, with just two electrons. As a result, helium does not form compounds with other chemical elements.
Helium Inert Elements
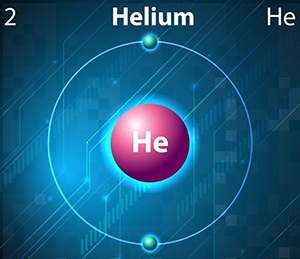
By contrast, hydrogen also has an outer shell capable of holding two electrons, but it actually has only one electron. For this reason hydrogen can form many compounds, including one which will be most familiar to readers, water (H2O), in which each of the hydrogen atoms gives up one electron, and the oxygen atom gains two electrons. In contrast, helium neither gives nor takes electrons, and therefore does not form compounds.
Is Helium Inert Gas
Related posts:
